Find the one stop pharma industry service provider that meets your needs at affordable prices.
Technology Verticals
Active ingredients, Formulation, Therapeutics, Cell therapy, Medical devices & Drug-Device Combinations

Regulatory Submission.
e-CTD, DMF, CMC. CSV, Validation & QMS services for NDA/ANDA/ IND/BLA and other details.

Electronic Batch Records
Batch records and quality control documents for manufacturing, packaging of pharmaceutical drugs & medical devices.
Knowledge Management Plus using AI-ML
Revolutionary 360 degree view of complete product development, clinical operations, technology transfer, validation, commercial launch and product lifecycle management using paperless KM+ platform.
Our Services

New Drug Application (NDA)
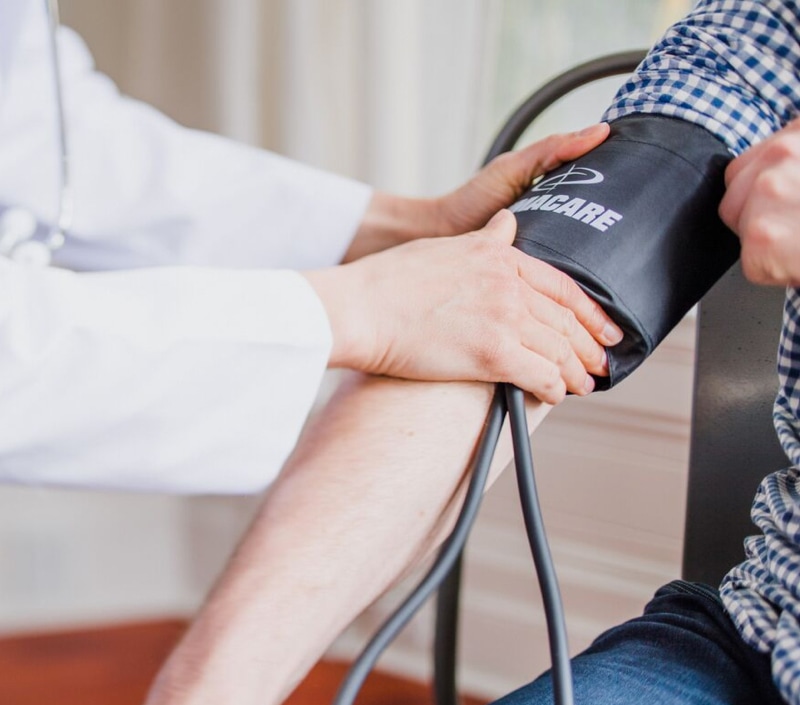
New Biologics Application (BLA)

Investigational New Drug (IND)

Generic Drugs (ANDA)

Medical Devices
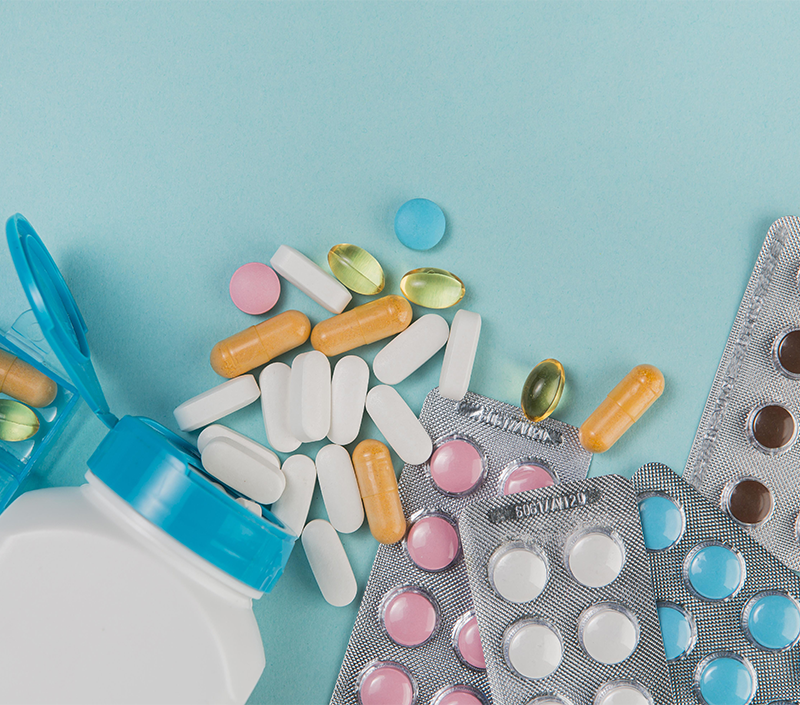
All Approved Drugs
Meets all your needs’
Related to pharma industry are met from conception to clinical PK/PD, regulatory filing and approval. Validation and launch, continous process improvement and life cycle management’
Pharmaceutical healthcare industry needs are met from concept to launch with FDA approved 3 step validation turn key service provider.
Contact Our Experts
For all your healthcare products contact our expert team members providing complete CMC [Chemistry Manufacturing and Quality Control] technical support.
